Immunizations and Vaccines
Infectious diseases can make people feel very sick or even die. Immunizations - or vaccines - are a safe and reliable way to fight these diseases.
Different vaccines work in different ways, but every vaccine helps the body’s immune system learn how to fight germs. They are proven to be safe and effective.
Vaccines are available through your doctor, at pharmacies, and at Alexandria Health Department clinics. Bring your immunization records to your appointment to make sure you get the correct shots.
Childhood and School-Required Immunizations
Virginia schools require immunizations for entry into kindergarten, 7th grade, 12th grade, and when transferring in. Failure to do so may impact a student’s ability to return to school in the fall.
A list of required school immunizations and related forms/documents are available on the Virginia Department of Health website.
Where to get a vaccine
Immunizations are available from your child's doctor, pharmacies, or clinics. They are also available through the health deprtment at our Main Office or our Teen Wellness Center Clinics. Details below.
| Main Office (all ages) | Teen Wellness Center (ages 12-19 only) |
|---|---|
| 4850 Mark Center Drive (4th Floor) | Alexandria City High School King Street & Minnie Howard Campus Locations |
| Call for appointments: 703.746.4888 | Call for appointments: 703.746.4766 |
Hours: Mondays: 8 a.m. - 12 p.m., 2 - 6:30 p.m. Tuesdays, Wednesdays: 8 a.m. - 4:30 p.m. Fridays: 8 a.m. - 12 p.m. | Hours: Monday, Tuesday, Wednesday, Friday: 9 a.m. - 5 p.m. Thursdays 1 - 5 p.m. |
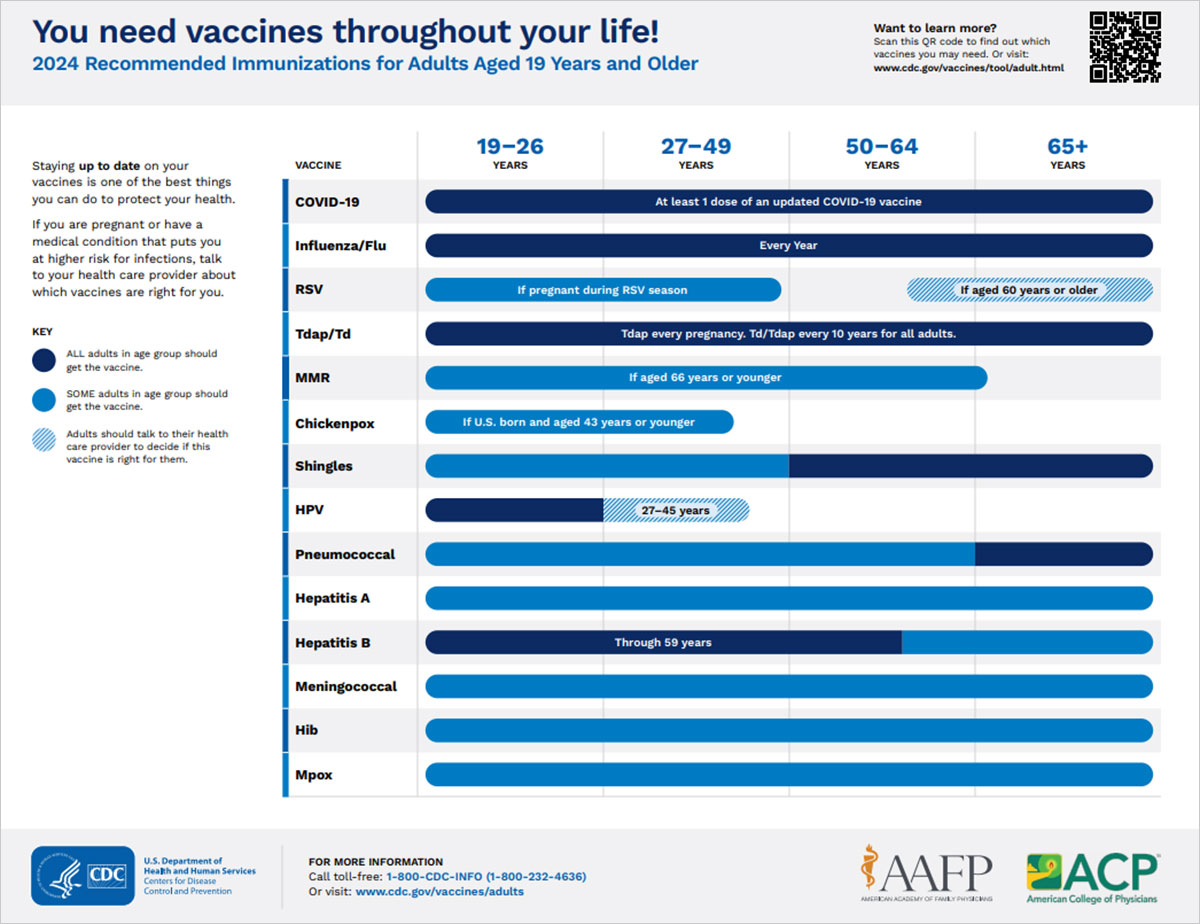
Adult Immunizations
Regardless of age, we all need immunizations to keep us healthy. With time, immunity from childhood vaccines can wear off and you may be at risk for new and different diseases. With adulthood comes responsibility, including the need to protect ourselves and our loved ones. Learn more from the Centers for Disease Control and Prevention.
Everyone should make sure they're up to date on these routine vaccines:
- COVID-19 vaccine
- Flu vaccine (influenza)
- Tdap vaccine (tetanus, diphtheria, and whooping cough) or Td vaccine (tetanus, diphtheria)
You may need other vaccines, too. Review the sections below to learn what other vaccines you may need based on:
- Age
- Life events, job, or travel
- Health conditions
- If you are pregnant or have a medical condition that puts you at higher risk for infections, talk to your health care provider about which vaccines are right for you.
Where Adults Can Get Vaccines
Vaccinations for adults are available from your health care provider, some pharmacies, and Alexandria Health Department's clinic at 4850 Mark Center Drive.
Bring all immunization records to your appointment.
Respiratory Illness Vaccines - COVID, Flu, & RSV
NOW is the best time to get vaccinated before respiratory viruses like flu, RSV, and COVID-19 start spreading more widely. Vaccines are now available to protect you and your family.
Please note: AHD currently has flu and COVID-19 vaccines for uninsured people only. AHD does not have RSV vaccines.
- Flu Vaccine
- What to know: It’s approved and recommended for everyone 6 months and older.
What to do: You can get it now from your doctor or a pharmacy. If you do not have health insurance, you can get this season's flu vaccine at a sliding fee scale by appointment only at Alexandria Health Department. Call 703.746.4888 for appointments.
- RSV (Respiratory Syncytial Virus) Vaccine
- What to know: It’s a one-time shot, approved and recommended for:
- Everyone 75 and older
- People 50–74 with certain health conditions (like chronic illness, severe obesity, weakened immune systems, or those living in nursing homes)
- Pregnant people who are 32–36 weeks along and due between September and January
- Some babies born during RSV season if their mother didn’t get the vaccine during pregnancy.
- What to Do: If you're in one of the groups above, talk to a doctor or pharmacy about getting an RSV vaccine. Always ask about costs - some Medicare plans do not cover the cost of the RSV vaccine.
- COVID-19 Vaccine
- What to Know: It's approved for everyone 6 months +. Ask your doctor or a pharmacist for the shot if you want it.
- The CDC has recommended people have individual conversations with a provider about getting the shot.
- Deciding if the shot is right for you or your child? The American Academy of Pediatrics, the American College of Obstetrics and Gynecology, and the American Academy of Family Physicians have collectively recommended the shot for every person age 6 months and older, including during pregnancy and breastfeeding.
- Where to Get It: Get it from your doctor or a pharmacy. If you do not have health insurance, you can get this season's COVID-19 vaccine at a sliding fee scale by appointment only at Alexandria Health Department. Call 703.746.4888 for appointments.
- Note on COVID-19 vaccines for children under 3 years old.
- Pharmacists cannot give COVID-19 vaccines to children under 3.
- Uninsured children can get vaccinated at AHD. Call 703.746.4888 to make an appointment.
- Insured children cannot get vaccinated at AHD. Parents should:
- Call their child’s doctor to ask if they offer the COVID-19 vaccine. (Some doctors may not have it this year due to cost.)
- Visit a CVS Minute Clinic, which offers vaccines for children 18 months and older).
- Note on COVID-19 vaccines for children under 3 years old.
Mpox (formerly known as monkeypox) Vaccines
Visit AHD's mpox page for information on places to get the mpox vaccine in Alexandria.
AHD Immunization Clinic Appointments
AHD immunization services are available by appointment only on a sliding fee scale.
Please Bring:
- Immunization records, if you have them
- Insurance information, if you are insured
- Optional: a completed registration form (you can complete this at AHD) English | Spanish.
Call 703.746.4888 for Appointments
Hours:
Monday: 8 a.m. - 12 p.m.; 2 - 6:30 p.m.
Tuesday: 8 a.m. - 4:30 p.m.
Wednesday: 8 a.m. - 4:30 p.m.
Friday: 8 a.m. - 12 p.m.
We do not offer immunization appointments on Thursdays or weekends.
Visit the Teen Wellness Center website for appointment info through TWC clinics.
Request Your Immunization Records
If you received immunizations at Alexandria Health Department:
- Request your immunization record by calling 703.746.4980.
If you received your immunizations somewhere else:
- Talk to your health care provider.
- For immunizations received in Virginia, request immunization records online or call 877-VAX-IN-VA.
- For immunizations received outside of Virginia, see CDC's tips for locating your immunizations records.
How Can I Receive More Information?
Call AHD's call center at 703.746.4988 from Monday - Friday, 9 a.m. to 5 p.m. for more information.